Spinal Cord Stimulation
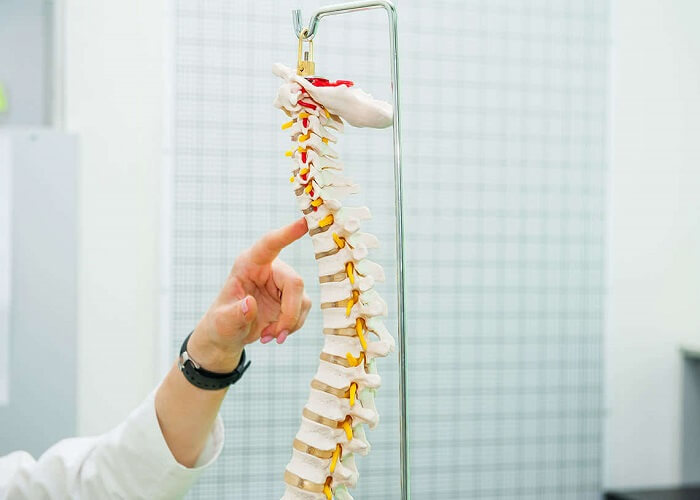
Spinal cord stimulation (SCS) describes the use of pulsed electrical energy near the spinal cord for control of pain. This technique was first applied in the intrathecal space and finally in the epidural space as described by Shealy, Mortimer, and Reswick in 1967. In the present day, most commonly, SCS involves the implantation of leads in the epidural space to transmit this pulsed energy across the spinal cord or near the desired nerve roots. This technique has notable analgesic properties for neuropathic pain states, anginal pain, and peripheral ischemic pain. The same technology can be applied in deep brain stimulation, cortical brain stimulation, and peripheral nerve stimulation (PNS).
History
A bump on the head often feels better when it is vigorously rubbed. In a similar fashion, the theories on neurostimulation began when this observation was analyzed physiologically. Neurostimulation began when Melzack and Wall’s published the gate control theory in 1965. This theory proposed that nonpainful stimulation of large myelinated A-beta fibers could impede painful peripheral stimuli carried by C-fibers and lightly myelinated A-delta fibers. Shealy, Mortimer, and Reswick designed the first spinal cord stimulator device for the treatment of chronic pain. Although this technique was noted to control pain, early devices did not garner interest for clinical application because of the impracticality of the required external power supply, which transmitted power transdermally via an internal coiled antenna.7 In the 1980s, implantable batteries were developed and spinal cord stimulators became a clinical option. Early indications included persistent neuropathic pain, spasticity, ischemic limb, and facial pain. The first rechargeable implantable pulse generators (IPGs) became available in 2004, which dramatically increased the stimulator lifespan. Deep brain stimulation was developed during the same time frame. In 2006, 14,000 new spinal cord stimulators were reported to be implanted annually. The market continues to grow rapidly. Although the gate theory was initially proposed as the mechanism of action, the underlying neurophysiologic mechanisms are not clearly understood.
Devices and Components
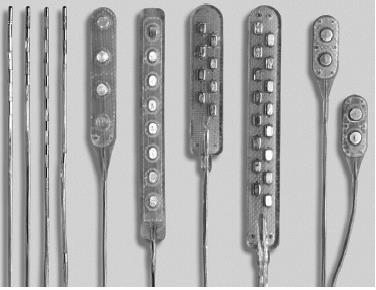
Two types of leads are used in spinal cord stimulators: paddle and percutaneous. Paddle leads are flat and wide, with insulation on one side and electrical pads on the other. This has the advantage of directing current in one direction. Paddles leads must be surgically placed via laminotomy or laminectomy. Percutaneous leads are cylindrical catheters placed via spinal needle. Because contacts are cylindrical, they generate an electric field circumferentially around the catheter.
IPGs are of two types: primary cell and rechargeable
-
- Primary cell devices have an average lifespan of 4 years, versus 9 years for rechargeable devices, but the lifespan is heavily dependent on usage. Primary cell devices tend to be larger but are useful for patients who need low outputs or do not recharge consistently. Small primary cell generators are available for pediatric or petite patients, but they are limited to low or infrequent currents.
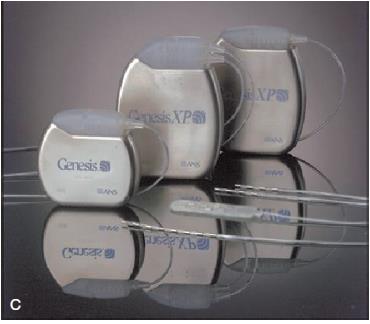
- Rechargeable IPGs contain Li-ion cells, which have a fixed number of charge-discharge cycles, with a degradation in the battery capacity over the span of multiple cycles. Most systems have a safeguard against full discharge because chemical changes can result in permanent battery failure. When patients fail to recharge the IPG, the generator fail safes to deep-discharge mode during which the device is disabled until reprogrammed.
Overall, battery life depends on the stimulation energy necessary (voltage/amplitude, number of active leads, pulse width, frequency), hours of usage (all day, during waking hours, intermittent doses), depth of discharge (interval and consistency of recharging), and battery degradation. Physicians need to understand how patients plan to use the stimulator to plan for the best generator option
Many projected innovations will continue to make SCS an attractive option for treatment of pain. Modern implants have a lifespan of 2 to 10 years, but battery capacity and microprocessor power consumption have improved rapidly, which will eventually prolong the lifespan, decrease the maintenance requirements, and reduce costs of future implantable devices.
Current stimulators are contraindicated for use within magnetic resonance imaging (MRI) because of the risk of magnetically generated currents heating the leads and causing neural injury; manufacturers are developing MRI-compatible leads. Research is evolving that demonstrates synergistic effects of intrathecal medications with SCS compatible leads, combined pump-stimulators. As the physiologic understanding of DC stimulation improves, newer modes of pulse waveforms and neuroanatomic distribution of currents can substantiate novel therapeutic roles. Closed-loop biofeedback innovations that record neural responses to SCS could play a role in improving the effects of SCS.
Conclusion
SCS is an invasive, interventional surgical procedure. Linderoth and Meyerson66 wrote some principles of neurostimulation that are cornerstones of SCS theory and practice. The difficulty of randomized clinical trials in such situations is well recognized. On the basis of the present evidence with two randomized trials, one prospective trial, and multiple retrospective trials, the evidence for SCS in properly selected populations with neuropathic pain states is moderate. Clearly, this technique should be reserved for patients who have failure with more conservative therapies. With appropriate patient selection and careful attention to technical issues, the clinical results are overwhelmingly positive.